The difference between the IEC 60825-1 test report of Amazon‘s laser products and the FDA 21 CFR test report
1. What is FDA 21 CFR test report
FDA 21 CFR is the US Food and Drug Administration‘s test standard for laser products. Laser products introduced into the United States or imported into the United States must:
Comply with applicable 21 CFR §1040.10 and §1040.11,
Certified and marked in accordance with 21 CFR §1010.2 and §1010.3, and reporting in accordance with 21 CFR §1002.10.
2. What certification is required to sell laser products on Amazon?
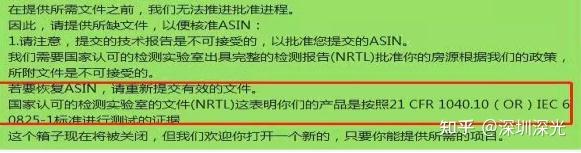
The laser product link on Amazon was taken off the shelf, and the email required to provide the test report of NRTL laboratory IEC60825 or 21CFR 1040.10. Deeplight can provide the report of NRTL laser product report.
3. What is the difference between the FDA CFR 1040.10 test report and the IEC60825-1 test report?
FDA CFR 1040.10 is the report on the testing standard of laser products in the United States, and IEC60825-1 is the report on the testing standard of laser products by the International Electrotechnical Organization. The laser products exported to the US market or sold on Amazon‘s US site are mainly subject to FDA report or certification, and the laser products exported to Europe or sold on Amazon‘s European site are mainly subject to IEC reports.
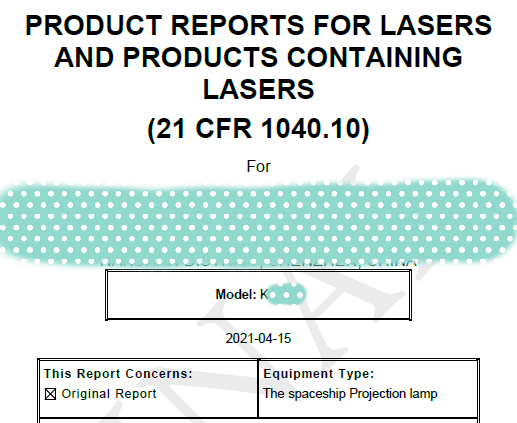

4. Classification, certification labels and grade labels of FDA laser products
1) When producing or selling laser products, it is often necessary to put a laser grade label on the package to remind users, but how is this grade distinguished?
Class I: Laser products with an output power of 0-0.4 mW have no biological hazard. Any possible viewing beams are shielded and the laser system is interlocked when the laser is exposed.
Class II: The output power of laser products is 0.4-1 mW. Will not burn the skin and will not cause a fire. Because eye reflections can prevent some eye damage, these types of lasers are not considered hazardous optical devices.
Class IIIa: Laser products with an output power of 1 mW to 5 mW. Does not burn the skin. Under certain conditions, these types of lasers can cause blindness and other damage to the eye.
Class IIIb: Laser products with an output power of 5 mW to 500 mW. At relatively high power, such laser products can scorch the skin. This type of laser product is clearly defined as hazardous to the eyes, especially at relatively high powers, which will cause eye damage.
Class IV: The output power of laser products is greater than 500 mW. These types of laser products can definitely cause eye damage. Like burning skin and igniting clothing, lasers can ignite other materials. High-power laser products basically belong to this category.

FDA grade label
2) Certification label
With the FDA statement: Complies with 21 CFR Chapter 1, Subchapter J. or Complies with 21 CFR 1040.10 and 1040.11. and the rating

5. Common products that require laser FDA certification
1. Components of audio, video and computer equipment such as (CD, DVD, Blu-ray, HD or other discs) players and recorders
2. Many barcode readers
3. Printers, copiers, fax machines
4. Laser pointer and pen for presentation, measurement and positioning
5. Optical fiber systems for telephone, video and computer networks.
6. Suitable for material processing operations such as cutting, welding, engraving or marking systems.
7. In the laboratory for research, measurement and light source applications.
8. Lasers specially designed for medical procedures.
9. Lasers specially designed and promoted for laser shows, entertainment, advertising, etc.
6. What information is required for the FDA test report or FDA certification of laser products
1. FDA authorization letter
2. User Manual
3. Maintenance Manual
4. Electrical schematic diagram, PCB Layout diagram
5. Product Labels
6. Certification label
7. Laser grade label
8. Laser Head Specifications
9. Block diagram of the electrical structure of the whole machine
10.IQC inspection procedures and records
11. Production line control procedures and records
12. QC, QA inspection procedures and records
13. Design reliability
14. Instruction manual and calibration certificate of the optical power meter
7. Amazon laser product registration number, Amazon FDA file registration number
After the laser product has been tested and obtained the report, it will receive a registration letter after submitting it to the FDA, and the registration letter will contain the registration number of the laser product.
8. The cycle and cost of applying for FDA certification or FDA test report for laser products
According to the product situation, under normal circumstances, it takes two weeks to test and submit to FDA for two weeks. The cost is mainly the test fee charged by the testing agency. Some products may also need to pay the FDA registration fee of about $5,000.
To inquire about the specific cost of your product, please contact Deeplight for an accurate quotation
Recommended items
-

Battery IECEN 62133
The IECEN 62133 standard is mainly aimed at the safety requirements of single batteries and batteries containing alkaline or non-acid electrolytes and portable sealed single batteries and batteries (including lithium batteries, Ni-MH batteries, Ni-CD batteries, etc.). It mainly includes the testing and verification of the following items:...View more -

Deeplight Technology OTA testing service of wireless products
TRP (total radiated power) and TIS (Total Isotropic Sensitivity) test methods and requirements. (1)TRP test requirements 1) EUT is transmitting at maximum power; 2) Non-portable EUT needs to be tested in free space; 3) Portable EUT test under the condition of human head model ......View more -

PTCRB and GCF certification tests for LTE Category M (eMTC) and NB-IOT
LTE Category M (eMTC, LTE Cat-M1) is the name of LTE-M (LTE-Machine-to-Machine) in 3GPP R13, and is an IoT technology based on LTE evolution. Known as LTE enhanced MTC (eMTC) in R13, it is designed to meet the needs of IoT devices based on existing LTE carriers....View more -

KDDI certification test
Unlike Japanese mobile phone users, Chinese mobile phone users like to freely choose a mobile phone on the market and then choose telecom operators such as China Mobile, Unicom and Telecom, while most Japanese choose a telecom operator first. and then choose a contract machine from this telecom operator, so most of the mobile terminals are sold by Japanese telecom operators. When Chines...View more




